

Tungsten boride (W2B5) is compound of tungsten and boron. Its most remarkable property is high hardness.
Basic Information:
English Name: Tungsten Boride
Chemical Formula: W2B5
CAS Number: 12007-98-6
Pub Chem: 6336862
Molar mass: 428.79100 g·mol
Appearance: Black Powder
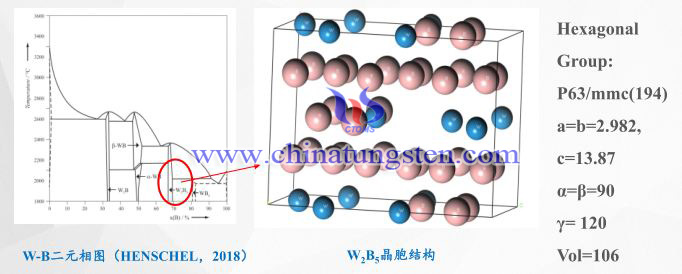
Tungsten boron can form a variety of tungsten boride compounds, and phase control is difficult;
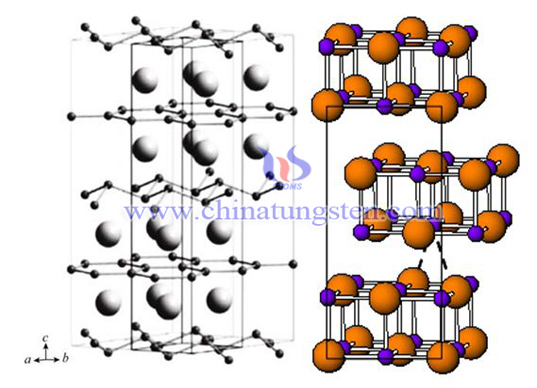
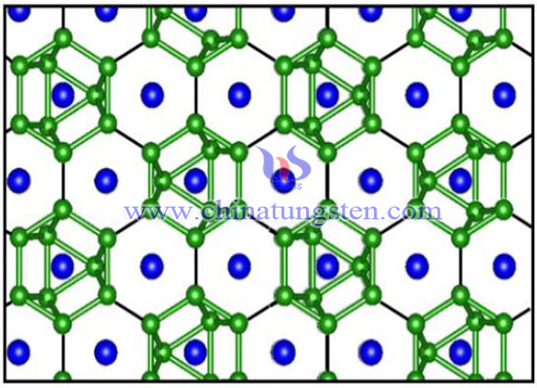
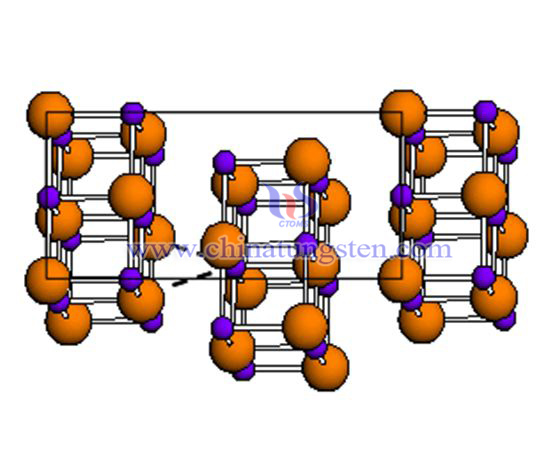
W2B5 is a hexagonal structure, tungsten and boron are connected by covalent bonds, and the structure is stable.


Tungsten Boride Specification
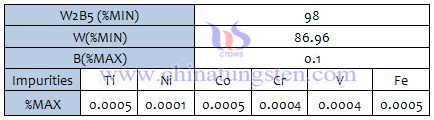
Tungsten Boride XRD
Tungsten Boride SEM


Fine Grain Size D99 < 38UM
Coarse Grain Size 100~150UM


High Hardness: 36~41GPa (Vickers hardness), tungsten carbide is 22~30GPa.
High melting point: 2365℃.
High electrical and thermal conductivity: electrical conductivity of 5.3×104S/cm, close to aluminum bronze alloy.
High chemical inertness: strong corrosion resistance, can be used as electrode anti-corrosion coating.
Good oxidation resistance: the long-term working temperature in the air is 800℃, and it can resist 1200℃ in short time.
With neutron shielding ability.


Compared with diamond and cubic boron nitride:
1. Compared with diamond, no carburization occurs during the processing process, and the oxidation resistance is better.
2. The synthesis conditions do not require high pressure, which reduces the cost and is suitable for mass production.
Compared with tungsten carbide:
1. Higher hardness;
2. Better oxidation resistance;
3. Better electrical and thermal conductivity;
4. With neutron shielding.


The method of direct sintering of tungsten powder and boron is commonly used. Other methods include the mixture of aluminum reducing tungsten dioxide and boron trioxide by molten salt electrolysis and vapor deposition.
Tungsten boride can be sintered at 1200 ~ 1300 ℃ in vacuum or argon with 2mol of tungsten and 5mol of boron powder. Reduction of tungsten trioxide with boron will also produce tungsten pentaboride, but at the same time it will produce tungsten monoboride as a by-product.


Applications of W2B5:
1. High temperature applications, e.g. crucibles
2. Thermal spraying powder
3. Crucibles for non-ferrous metals
4. Manufacture of cutting tools
5. Ballistic protection
6. Superhard material additive
7. Material intermediate
A patent claims that a workpiece with a tungsten rich boride coating includes a workpiece substrate, a tungsten layer, a tungsten boride gradient layer and a tungsten rich boride coating arranged on the substrate in turn; The boron content increases gradually along the workpiece substrate to the thickness direction of tungsten layer and tungsten boride gradient layer. Application of tungsten boride metal/polymer matrix composites in the field of nuclear shielding materials: nuclear shielding materials usually require the addition of neutron and gamma ray shielding components at the same time to achieve simultaneous shielding of neutrons and γ Function of ray.
In addition, it is believed that the component distribution is uniform, the mechanical properties are excellent, and both neutrons and γ High bulk density tungsten boride aluminum matrix composites, tungsten boride titanium matrix composites, tungsten boride polymer matrix composites with ray shielding properties. The thermal neutron shielding efficiency of 3 mm thick tungsten boride aluminum matrix composite reached 99%; 15cm thick tungsten boride aluminum matrix composite γ The shielding rate of ray (0.662; MeV) reaches 99%. The new type of tungsten borate nuclear radiation protection composite is expected to be widely used in the fields of spent fuel storage, aerospace, reactor shielding, instrument components, medical radiation protection, etc.


Chinese Name: 一硼化钨、硼化钨
CAS:12007-09-9
Molecular Weight:194.65
Melting Point: 2860 ℃
Tungsten monoboride and tungsten boride (WB) are gray orthorhombic powder with silver white octahedron. Tungsten boride and tungsten boride are characterized by high hardness, high toughness, wear resistance, high temperature resistance, corrosion resistance, good chemical stability and conductivity, and excellent neutron absorption effect. Tungsten boride (WB) does not react with hydrochloric acid, but can react with hot sulfuric acid and nitric acid. It is insoluble in water and soluble in aqua regia, especially in the presence of hydrofluoric acid. It is soluble in aqua regia and some concentrated acids and can be decomposed by chlorine at 100 ℃. Tungsten boride will decompose in natural environment, so it is generally stored in a closed, cool and dry environment.
Uses of tungsten boride and tungsten boride: tungsten boride is commonly used as structural materials, wear-resistant materials, refractory materials, electrode materials, semiconductor films, cutting tool materials, corrosion resistant materials, etc. Boracia tungsten is also used as chemical reagent, fine chemicals, pharmaceutical intermediates, material intermediates, etc.
Preparation method of tungsten boride and tungsten boride: at 1900 ° C, tungsten boride can be converted into tungsten boride by heating it with carbon. It can be made by direct reaction of tungsten and boron heated by electric furnace.

中文名称:硼化二钨
Chemical Formula: BW2
Molecular Weight: 378.49
CAS Number: 12007-10-2
Density:1.6(g/mL,25/4℃)
Melting Point: 2770 ℃.
Tungsten diboride (W2B) is a gray black square crystal powder with metal conductivity, insoluble in water and soluble in aqua regia. Tungsten boride can be reduced to tungsten metal with titanium, tantalum and zirconium.
Tungsten diboride has high melting point, high hardness, high conductivity and high corrosion resistance and oxidation resistance to different types of media, which make W-B series compounds widely used in harsh environments. For wear-resistant coatings and semiconductor films, high-temperature corrosion resistant electrode materials, casting molds, crucibles, etc; It is also commonly used for wear-resistant coating of wear-resistant parts and semiconductor film.
Synthesis of Tungsten Diboride
(1)Tungsten and boron are used as raw materials. They are fully mixed, ground, heated and reacted above 1400 ℃ to obtain tungsten boride, and then continue to react to generate W2B. After cooling and crushing, the product is obtained.
(2)Tungsten and stoichiometric boron powder are mixed at a molar ratio of W ∶ B=2:1 and sintered at 1200 ~ 1300 ℃ in vacuum or argon atmosphere to produce tungsten diboride.
(3)Solid phase method is adopted. Tungsten boride is prepared with metal tungsten and boron as raw materials. The reaction formula is as follows. The stoichiometric metal tungsten powder and the element boron are fully mixed, ground, shaped, and heated to react at 1400 ℃ to obtain tungsten boride. Continue the reaction to generate W2B, and obtain the product after cooling and crushing.
Application of Tungsten Diboride
For wear-resistant coating and semiconductor film of wear-resistant parts.
Storage of Tungsten Diboride
Store in a closed, cool and dry environment. It will not decompose if used according to regulations.
Product Classification of Tungsten Diboride
Nanometer tungsten boride, nanometer tungsten boride

Melting Point: 2900℃。
Boron Tungsten (WB2) is a silver white octagonal crystal. WB2 is insoluble in water and soluble in aqua regia.
Boron Tungsten Producing Method
Boron and tungsten powder are co heated to high temperature to interact.
Boron Tungsten Ceramic (WB2)
The transition metal boride WB2 has a higher hardness, ≥ 40 GPa, and is a super hard material. As a transition metal boride, WB2 ceramics should have excellent properties of boride, which can be used as an important functional material in the fields of surface treatment and mechanical manufacturing. B-W series has several intermediate compounds, such as W2B, WB, WB2, W2B5, WB4, WB12, etc, The phase composition of the synthesized tungsten boride is complex.
WB2 is a boride of transition metal, which has high melting point, high hardness, high conductivity and excellent wear resistance. It has high corrosion resistance and oxidation resistance to different types of media, making it widely used in high-temperature structural materials, refractory materials, electrode materials and other fields. For example, ZrB2 is used as the protection tube of thermoelectric electrode and thermocouple to measure the temperature of molten steel and iron; TiB2 is used as the cathode material and coating with high corrosion resistance for aluminum electrolysis production; HfB2 is used as ultra-high temperature material for high-speed space rocket; MgB2 has attracted much attention as a new type of superconducting material because of its excellent superconductivity.
A Preparation Method of Boron Tungsten:
1. The tungsten powder and the amorphous boron powder are mixed to form a reaction powder at a ratio of 1:2 ‑ 3; 2. Mixing reaction powder and molten salt to obtain mixed powder; 3. The mixed powder is heat treated under vacuum or inert gas protection at a temperature of 1000 ‑ 1300 ℃. After heat treatment, it is naturally cooled to room temperature to obtain a mixture of tungsten diboride and molten salt; 4. The mixture of tungsten diboride and molten salt is separated to obtain tungsten diboride.
Boron Tungsten Grinding Paste
With the progress of science and technology, people have higher and higher requirements for the hardness of materials It is of great significance to quickly obtain the hardness of newly synthesized samples for the discovery of new materials. When testing the hardness of samples, it is necessary to ensure the flatness of the sample surface. Therefore, polishing technology is the key factor to limit the hardness test cycle, and tungsten diboride grinding paste has a good guarantee for the polishing quality of hard materials.


Synthesis method of tungsten triboride WB3: 200 mesh tungsten powder with purity of 99.95% and amorphous boron powder with purity of 99.99%, mix tungsten powder and boron powder in agate mortar for more than 3 hours; The mixed powder is pressed and formed by the powder forming voltage machine, and then the products are packed into the composite block by the boron nitride tube wrapping group, which is hydraulically pressed at the top of the hexahedron (the pressure is 5GPa), the temperature is 2000 ℃, and the high temperature and high pressure sintering is carried out. The heat preservation and pressure holding time is 15min.
Atomic Structure of Tungsten Tetraboride and Tungsten Triboride
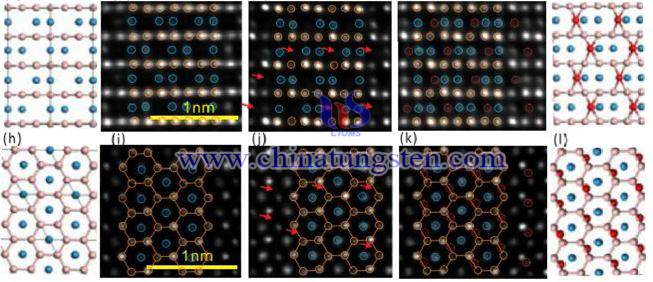
Relevant research found that WB3 has tungsten atom deletion, and the real structure should be W1-xB3, which is a mixture of W1-xB3 and WB3, but the main structure is still WB3. In the same industry, many studies have confirmed that WB4 should be a WB3 structure. Because WB3 is a metastable phase, the edge of its grain is easy to form tungsten atom deletion. The experimental results show that the hardness convergence value of WB3 is 25.5 GPa.
Researchers use Ac HRTEM to discover WB3
Materials with Vickers hardness greater than 40 GPa are called superhard materials; Conventional superhard materials are diamond (70-120 GPa) and cubic boron nitride (>50 GPa). The combination of transition metals with high charge concentration and boron atoms that can form strong covalent bonds is considered to be two important factors that can form new superhard materials. In theory, superhard materials mainly exist in two major compound systems: the first is the simple substances and their compounds of boron, carbon, nitrogen and oxygen; The second category is compounds of transition metal elements and light elements (boron, carbon, nitrogen). Therefore, WB4 and WB3 have also attracted people's attention as superhard materials.


Tungsten tetraboride WB4 is hexagonal structure, and its main structure is α- The structure of Mo B2 is similar, but the main difference is that there are two more boron atoms in the W layer, forming a boron boron dimer. This boron boron dimer connects graphene like boron layers in WB4 to form a three-dimensional boron structure. It is believed that among these transition metal borides, WB4 was once considered as a potential superhard material due to its 3D boron network structure, but its Vickers hardness is only close to 30 GPa, which is not superhard material; Theoretical calculation shows that the structure of WB4 is unstable in dynamics, mechanics and energy. However, WB3, which lacks the layered structure of boron boron dimer, is stable. Therefore, theoretical calculations suggest that WB4 does not exist, and WB4 should be WB3.
Composition description of WB4 composite matrix material applied by an American company in China
Transition metal borides have high hardness, wear resistance, good thermal stability and can be synthesized under normal pressure. They can be used as anti-wear coatings on machining tool materials and mechanical components such as cutting, grinding, polishing, etc. Among them, tungsten boride is favored due to its relatively low cost of raw materials. The hardness of WB4 is the highest among tungsten borates. However, WB4 will decompose into WB2 with lower hardness when the temperature is higher than 1000 ℃, thus reducing the mechanical properties.
W-B binary system compounds have high melting point, high hardness, high conductivity and excellent wear resistance. They also have excellent properties such as oxidation resistance and high corrosion resistance for different types of media. These excellent properties enable W-B binary system compounds to be widely used in harsh environments, such as high-temperature corrosion resistant electrode materials, melting casting molds, crucibles, etc. In addition, boron is a good neutron absorber because of its high neutron absorption cross section and wide neutron absorption energy range γ The boron tungsten compounds have both neutrons and γ Comprehensive radiation shielding performance. Among W-B series compounds, tungsten tetraborate (WB4) is considered as a potential superhard material, so the research in this field has high scientific research value and practical value.
At present, there are few reports on the preparation and properties of WB4 at home and abroad. It is mainly because the boron element is easy to volatilize during the synthesis of B-W binary system compounds, and the stoichiometry of the obtained target product deviates, so it is difficult to synthesize WB4 with high purity and uniform particle size. Arc melting is now the main method for preparing tungsten tetraborate (WB4), but this method requires high synthesis conditions, and the vacuum degree and cooling rate in the furnace will also affect the quality of products.
A preparation method of tungsten tetraboride ceramic powder:
1) Mixing tungsten powder and boron powder to obtain mixed powder
2) The mixed powder is placed in a graphite hot pressing die and placed in a reaction furnace
3) After vacuuming the reaction furnace, raise the temperature to 1200~1600 ℃, and then heat it for 30~180min at a pressure of 10~100MPa; After the heat preservation is completed, the pressure is relieved and naturally cooled to room temperature to prepare tungsten tetraboride block
4) Remove the graphite paper on the surface of the block, and then grind the block to obtain tungsten tetraborate ceramic powder.

Tungsten pentaboride (WB5) is called a new boron rich phase, and it is also a future material to be developed. Its hardness and fracture toughness are superior to many existing materials. It can be prepared under normal pressure. Its key parameters, such as hardness and fracture toughness, are also more superior to many existing materials, such as artificial diamonds and hard tungsten carbide cobalt composites. Therefore, WB5 has good application prospects in drilling, machining, national defense and other fields.
According to the calculated composition temperature phase diagram, scientists predict that the new boron rich compound WB5 will be super hard, with a Vickers hardness of 45GPa, a high fracture toughness ≈ 4 MPa • m0.5, and maintain thermodynamic stability at a wide range of temperatures under environmental pressure. The temperature dependence of the mechanical properties of boron rich WB3 and WB5 phases was studied using quasi harmonic and non harmonic approximation. The results show that WB5 is still a high performance material even at very high temperature.

中文名称:五硼化二钨
CAS Number: 12007-98-6
Molecular Formula:428.79
Exact Weight: 430.00
Melting Point:210-214°C
Appearance: Black Powder
Production Method
2 mol of tungsten and 5 mol of boron powder can be sintered at 1200~1300 ℃ in vacuum or argon atmosphere; The reduction of tungsten trioxide by boron will also produce tungsten pentaboride, but tungsten monoboride will also be produced as a by-product. Tungsten borate is a special compound, which has potential applications in superhard materials, antifriction materials and catalytic materials. Tungsten pentaborate can be used as pharmaceutical intermediates or superhard materials. The results show that WB5 is still a high performance material even at very high temperature.
According to the calculated composition temperature phase diagram, scientists predict that the new boron rich compound WB5 will be super hard, with a Vickers hardness of 45GPa, a high fracture toughness ≈ 4 MPa • m0.5, and maintain thermodynamic stability at a wide range of temperatures under environmental pressure. The temperature dependence of the mechanical properties of boron rich WB3 and WB5 phases was studied using quasi harmonic and non harmonic approximation. The results show that WB5 is still a high performance material even at very high temperature.


中文名称:硼钨酸d
Chemical Formula: W₃(BO₃)₂,B2O3•(WO3)9•24H2O 。
Borotungstic acid is a light yellow liquid with a relative density of 3.00. Soluble in water and alcohol.
It is prepared by co heating ammonium borotungstate and aqua regia, and is used to determine the relative density of minerals.
Synthesis of borotungstic acid:
12WO42- +5NH4+ +BO33- +22H+ =(NH4)5[B(W3O10)4]•6H2O(precipitate)+5H2O。


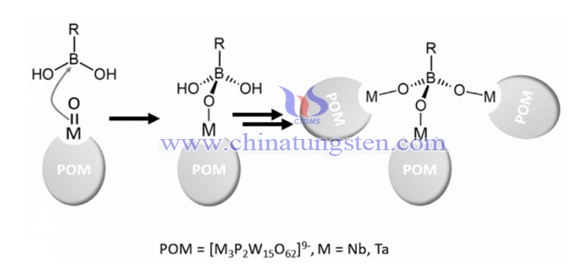
Borotungstate is a kind of common polyoxometalates POMs, including K7 [MBW11 (H2O) O39] • H2O (MBW11, M=Cu, Co, Ni) and K8 [BW (11) O (39) H] • 13H2O. The research on polyoxometalates (POMs) has gone far beyond the traditional classical structures such as Keggin and Dawson. The constituent elements have expanded from rich multielements such as Mo, W and V to nearly 100 species covering the periodic table of elements. There are also many kinds of borotungstate produced. A certain research has produced 23 related tungstate borotungstate derivatives:
KH2[Ce(H2O)8][Ce(H2O)3][α-BW11O39]•14H2O (1),
K4Na4H4[Pr(H2O)3]2 [α-BW11O39]2•30H2O (2) ,
KH3[Nd0.5K0.5(H2O)8][Nd(H2O)3][α-BW11O39]•14H2O (3) 和
Na K6H5{[W5O18]Ln[BW11 O39]}•22H2O [Ln = Sm3+ (4)、Eu3+ (5)、Dy3+ (6)、Ho3+ (7)、Er3+ (8)、Yb3+ (9)]。
Boric acid reacts with poly acid to produce OB-POMs
A series of organic and inorganic hybrid rare earth substituted borotungstate derivatives K4Na4H4 [Ln2 (gly) 4 were obtained by introducing organic ligands into the system in which borotungstate and rare earth ions exist( α- BW11O39)2]•23H2O [Ln =Ce3+ (10)、Pr3+ (11)、Nd3+(12)、Sm3+ (13)、Eu3+ (14)、Tm3+ (15)]。 A novel nanoscale polynuclear Ce4+ion substituted bismuth tungstate Na16 (NH4) 10H5 {(W14Ce6O61) [W3Bi6Ce2.5Na0.5 (H2O) 3O14 was synthesized by self-assembly reaction of simple raw materials( α- Bi W9O33)3]2}•38H2O (16)。 Compound (16) can be prepared by using Na2WO4 • 2H2O, Na Ac • 3H2O, Bi (NO3) 3 • 5H2O and (NH4) 2Ce (NO3) 6 in weakly acidic aqueous solution. Two kinds of bismuth tungstate dimers containing rare earths, Na2Li10 [Pr (H2O) 2 (Ac) Bi2W21O73] 2 • 36H2O (17) and Na2Li10 [Ln (H2O) (Ac) Bi2W21O73] 2, bridged by acetic acid, were obtained by one-step self-assembly reaction •54H2O[Ln = Eu3+ (18)、Gd3+ (19)、Tb3+(20)、Dy3+ (21)、Ho3+ (22)、Er3+ (23)]。 Among them, K8 is available for (1) - (3)[ α- BW11O39H] • 13H2O precursor, Na Ac • 3H2O and Ln (NO3) 3 • 6H2O are obtained by reacting in acidic aqueous solution medium, and (4) - (9) can be obtained in KCl solution by using simple raw materials Na2WO4 • 2H2O, H3BO3 and Ln (NO3) 3 • 6H2O. Compounds (10) – (15) can pass K8[ α- BW11O39H] • 13H2O precursor, Na Ac • 3H2O and Ln (NO3) 3 • 6H2O were reacted in aqueous solution and synthesized in the presence of organic ligand glycine. Compound (17) (23) was synthesized from Na2WO4 • 2H2O, Bi (NO3) 3 • 5H2O and Ln (NO3) 3 • 6H2O in Li Ac buffer solution. These compounds (17) - (23) represent the first organic-inorganic hybrid acetic acid bridged rare earth substituted bismuth tungstate derivatives, and they all contain two dimers formed by the cationic connection of [Bi2W21O73] 8-building units through metal organic ligands.
Borotungstate and bismuth tungstate compounds (all belong to triclinic system) are composed as follows:
1. KH2[Ce(H2O)8][Ce(H2O)3][α-BW11O39]•14H2O
2. K4Na4H4[Pr(H2O)3]2[α-BW11O39]2•30H2O
3. KH3[Nd0.5K0.5(H2O)8][Nd(H2O)3][α-BW11O39]•14H2O
4. Na K6H5[W5O18]Sm[BW11O39]•22H2O
5. Na K6H5[W5O18]Eu[BW11O39]•22H2O
6. Na K6H5[W5O18]Dy[BW11O39]•22H2O
7. Na K6H5[W5O18]Ho[BW11O39]•22H2O
8. Na K6H5[W5O18]Er[BW.11O39]•22H2O
9. Na K6H5[W5O18]Yb[BW11O39]•22H2O
10. K4Na4H4[Ce2(gly)4(α-BW11O39)2]•23H2O
11. K4Na4H4[Pr2(gly)4(α-BW11O39)2]•23H2O
12. K4Na4H4[Nd2(gly)4(α-BW11O39)2]•23H2O
13. K4Na4H4[Sm2(gly)4(α-BW11O39)2]•23H2O
14.K4Na4H4[Eu2(gly)4(α-BW11O39)2]•23H2O
15. K4Na4H4[Tm2(gly)4(α-BW11O39)2]•23H2O
16.Na16(NH4)10H5{(W14Ce6O61)[W3Bi6Ce2.5Na0.5(H2O)3O14(α-Bi W9O33)3]2}•38H2O
17. Na2Li10[Pr(H2O)2(Ac)Bi2W21O73]2•36H2O
18. Na2Li10[Eu(H2O)(Ac)Bi2W21O73]2•54H2O
19. Na2Li10[Gd(H2O)(Ac)Bi2W21O73]2•54H2O
20. Na2Li10[Tb(H2O)(Ac)Bi2W21O73]2•54H2O
21. Na2Li10[Dy(H2O)(Ac)Bi2W21O73]2•54H2O
22.Na2Li10[Ho(H2O)(Ac)Bi2W21O73]2•54H2O
23. Na2Li10[Er(H2O)(Ac)Bi2W21O73]2•54H2O

Pyrimidine Borotungstate (PBT) has good anti-tumor activity both in vivo and in vitro, and belongs to a low toxic compound. The mechanism of action may be: inhibit the DNA synthesis of tumor cells by affecting nucleic acid metabolism; The tumor cell cycle was arrested in G0/G1 phase; Induce tumor cell apoptosis to inhibit tumor cell growth and achieve anti-tumor effect; It can stimulate the activity of immune cells, protect immune organs, and enhance the immune function of the body, thus indirectly playing an auxiliary role in anti-tumor. PBT is a low toxic substance with good anti-tumor activity in vivo and in vitro; PBT can significantly inhibit the growth of human liver cancer SMMC-7721 cells, human gastric cancer SGC-7901 cells, and human cervical cancer HeLa cells in vitro, showing good anti-tumor activity. Among them, PBT has a strong killing effect on human liver cancer SMMC-7721 cells, and the inhibition effect is particularly significant. It is a new anti-tumor drug with great development potential.
In 2004, it was reported that pyrimidine borate tungstate (PBT) (fluorouracil keggin structure heteropoly tungstate) had anti-cancer activity. Many researchers designed and synthesized some heteropoly compounds according to the characteristics of heteropoly compounds and biological small molecules such as amino acids, pyrimidines and dipeptides to form inorganic organic hybrid compounds with certain biological activity. The anticancer effect of heteropoly acid salts in vitro is most in keggin structure, and the study of anderson structure, dawson structure and non classical structure is less. keggin structure has a good inhibitory effect on a variety of cancer cells, with low toxicity; The main research contents were acute toxicity, solid tumor inhibition rate (tumor weight), body weight, survival time, thymus index and spleen index of mice; The research on the anticancer biological activity mechanism of polyoxometalates mainly includes redox mechanism and apoptosis induction mechanism. However, the research on the anticancer biological activity of polyoxometalates inside and outside the country still has high synthesis cost, large toxic and side effects, low output, unclear mechanism of action, mostly reasoning research, lack of practical evidence, etc.
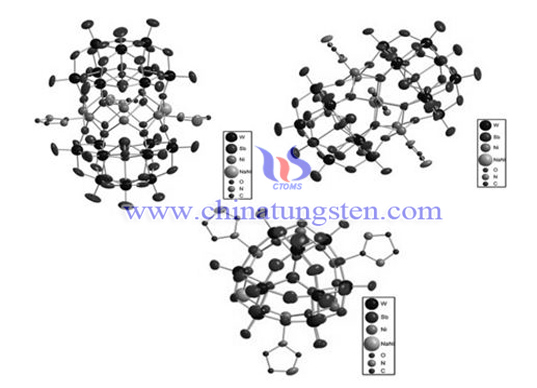
According to the invention, the molecular weight of the imidazole covalently coordinated Sandwich type polymetallic tungstate is 5672.4, belonging to monoclinic system, space group C2/c, its chemical formula is (Na0.7Ni5.7 (C3H4N2)} 3 (SbW9O33) 2] • 28H2O), and the cell parameter is α= 90°, β= 114.014°, γ= 90°,Z=4。 sandwich type polymetallic tungstate with imidazole covalently coordinated by self-assembly showed good anti-tumor activity, especially for liver cancer, lung cancer, gastric cancer and other tumor cells with high cytotoxicity. Synthesis method of imidazole covalently coordinated Sandwich type polymetallic tungstate: dissolve antimony tungstic acid in water and heat it to 80 ℃, add NaOH to adjust Ph to 8.9, then add NiCl2 • H2O, and add imidazole solution. The molar ratio of antimony tungstic acid, imidazole, and nickel salt is 1.5:4.9:5.8. Adjust pH to 4.7 with HCl acidification, reflux for 8h, filter, and grow orange crystal [Na0.7Ni5.7 (C3H4N2)} 3 (SbW9O33) 2] • 28H2O after several days of standing. See the structure diagram, and the yield is 57%.

Chinese Name: 钨硼酸镉、硼钨酸镉
Chemical Formula: B2Cd5H36O98W24
Melting Point: 75℃
Molecular Weight: 6600.06
Density: 3.28。
Cadmium tungstoborate is a yellow triclinic crystal. Stable in air. It can decompose under sunlight and is slightly soluble in ethanol.
Cadmium tungstoborate is toxic; Very soluble in water, more soluble in hot water, the solution is yellow or light brown.

Chinese Name: 硼钨酸钾
Chemical Formula: K5BW12O40• xH2O
Polyaniline doped with potassium 12 borotungstate was prepared by chemical oxidation polymerization with ammonium persulfate as oxidant. Polyaniline co doped with potassium 12 borotungstate (K5BW12O40onH2O) and hydrochloric acid was prepared. Through FT-IR, XRD and conductivity tests.


Chinese Name: 十八水合硼钨酸镉
Chemical Formula: Cd5(BW12O40)2•18H2O
Molecular weight 6600.25, yellow triclinic crystal, relative density 3.28, melting point (℃): 75. Very soluble in water, more soluble in hot water, the solution is yellow or light brown.


Chinese Name:硼钨酸镧
Chemical Formula: LaBWO6
LaBWO6 crystal of lanthanum borotungstate consists of LaBWO6, Li2W2O7, B2O3 and other powders and is prepared by molten salt method.
Lanthanum borotungstate red phosphor: the activated ion is trivalent rare earth europium ion Eu3+, the molecular formula is La1-xEuxBWO6, where x is the mole percentage of Eu3+doping, 0.0001 < x ≤ 0.5; The phosphor has strong excitation light near 400nm and 450nm, and the red light emission wavelength is mainly 616nm and 696nm, meeting the requirements of white LED.


Chemical Formula: La3(1-x)BWO9:3xBi3+
Bismuth doped tungstoborate yellow phosphor (La3 (1-x) BWO9: 3xBi3+) can be effectively excited by ultraviolet light in the range of 250 410nm. Its luminescence is in the range of 400 800nm, and its center is at~560nm. The excitation and emission are adjustable. It is a yellow phosphor for white LED. The ultraviolet near ultraviolet LED chip (350-410nm) is combined with red, green, blue or yellow and blue phosphors to produce white light. Several kinds of phosphors are coated on the UV LED chip. The chip excites the phosphors to form different colors of light, and white light is obtained by superposition of different colors of light. This scheme can obtain white light with high color rendering, small color difference and adjustable color temperature, overcoming the problems faced by the combination of blue LED chip and YAG: Ce phosphor. The new scheme requires that red, green, blue or yellow and blue phosphors must have absorption at 350-410nm, have no absorption in the visible light area, and emit light efficiently in the visible light area. Among the existing yellow phosphors, most of them use rare earth ions (Eu2+or Ce3+) as the activator, such as BaAl2O4: Eu2+, Sr8ZnSc (PO4) 7: Eu2+, LaSr2AlO5: Ce3+. In addition to absorption in the ultraviolet region, there is also strong absorption in the blue light region. The luminescence of some blue phosphors is reabsorbed by the yellow phosphors, leading to lower luminous efficiency.
The preparation method of bismuth doped tungstoborate yellow phosphor is to weigh raw materials according to the general chemical formula, grind and mix evenly; Presintering at 400 800 ℃ for 412h, cooling to room temperature, grinding and mixing; Then calcine at 1000 1300 ℃ for 412h, cool down in the furnace to room temperature, and grind to obtain bismuth doped tungstoborate yellow phosphor.


The research shows that the glass phase separation zone made of lithium borotungstate (Li2O-B2O3-WO3) is composed of two parts, one part is located at the periphery of the glass forming zone, which is an area with strong phase separation tendency, and the other part is located in the uniform glass forming zone. Phase separation glass is obtained after heat treatment. The glass phase separation tendency increases with the increase of WO3 and Li2O content. Lithium borotungstate (Li2O-B2O3-WO3) system glass forming zone can be used to produce composite conductive glass and ionic conductive glass with high conductivity in the acidic and alkaline zone respectively. Lithium borotungstate glasses have a strong tendency of phase separation. After phase separation, the continuous phase is rich in boron and the second phase is rich in tungsten. The addition of strong electrolyte salt will aggravate the phase separation of glass, and the addition of glass forming oxide can effectively inhibit the phase separation tendency of glass. The reason for phase separation is that tungsten ions have high field strength and tend to form relatively independent structures and polymerize into large groups.


Heteropoly acids, as a new environmentally friendly solid catalytic material with oxidation and strong acidity, are widely used in catalysis, material science, electrochemistry and other fields. Although heteropoly acids have many excellent characteristics, simple heteropoly acids are often easily soluble in water and strong polar organic solvents, and have low specific surface area and thermal stability. In order to overcome these shortcomings, some cations (K+, Ag+, etc.) can be introduced through modification to prepare stable and insoluble heteropoly acid solid catalytic materials. For example, potassium substituted borotungstic acid catalytic materials are an option.
Potassium borotungstate (K8 [BW11O39H] • 13H2O) has an irregular block structure of aggregates and a certain acidity. Potassium borotungstate has an irregular lumpy structure with uneven surface and relatively large specific surface area, which increases the contact between the catalytic active site and reactants and improves the catalytic activity; The post treatment process of potassium borotungstate catalyst is simple, easy to recycle and reuse, and its catalytic activity is still stable after repeated use for three times.


Chinese Name: 硼钨酸钡
Chemical Formula: Ba5H8[B(W2O7)6]2•xH2O
CAS#:1303-79-3
Molecular Weight: 6472.43
Appearance: White powder or crystal, soluble in water, easy to disintegrate into crystal powder in air, used for the preparation of other borotungstate.


Boron tungsten heteropoly acid catalyst contains peroxyborotungstic acid, which maintains the basic skeleton of Keggin structure, is a microporous crystal structure, and the molecular formula is C19H44W3BNO18
The combination of tungsten containing heteropoly acid salt and phase transfer catalyst has high reaction selectivity. This kind of catalyst can mainly react with hydrogen peroxide to generate [W (O2)] ternary ring containing active oxygen, which is dissolved in the reaction system under the action of quaternary ammonium salt cation for catalytic epoxidation in homogeneous phase, improving the reaction efficiency; In addition, when the hydrogen peroxide is exhausted, the catalyst will be separated from the oil-water two-phase interface in solid form, thus solving the problem of separation of conventional acid catalyst.


Polyoxometalates (POMs), also known as metal oxygen cluster compounds, are widely concerned for their diversity in species and structures and potential applications in catalysis, medicine, material science and other fields. The oxygen atoms (terminal oxygen or bridge oxygen) on the surface of polyanions have the ability to coordinate, and the metal groups can be bonded to their surface skeletons to form new compounds with one-dimensional chain, two-dimensional layered and three-dimensional network structures using polyoxometalates as basic building units.
Keggin type boron tungsten copper heteropoly acid salt/polyamide amine multilayer composite film was prepared in situ on indium tin oxide electrode by electrostatic layer by layer self-assembly method. CuBW11 assembled into the multilayer film retained good electrochemical properties in aqueous solution, and the multilayer composite film formed by alternating assembly with PAMAM was flat and evenly distributed on the electrode surface, The reduction reaction of nitrite ion in acidic solution shows good catalytic activity and the stability of the membrane is good, which is conducive to the realization of heterogeneous electro catalytic reaction. Therefore, these films are expected to have practical application value in electrochemical analysis, new electrocatalytic materials and other fields.
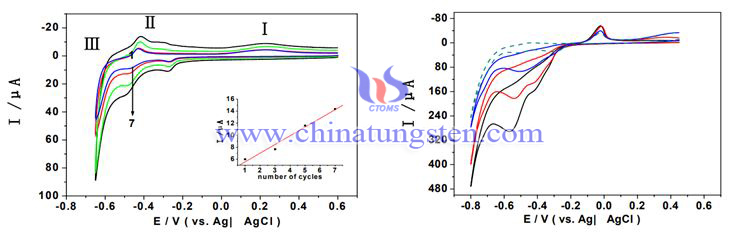
Keggin type boron tungsten copper heteropoly acid salt/polyamide amine multilayer composite film was prepared on indium tin oxide electrode. The experiment proved that the multilayer film modified electrode had good stability in acid solution and good electrocatalytic activity for nitrite, etc.
Borotungstic heteropoly acid salts, which show excellent characteristics in alcohol oxidation, are used as catalysts. The boron tungsten heteropoly acid quaternary ammonium salt with Keggin structure is kept, and the strong acid phosphoric acid in liquid is replaced by solid weak acid boric acid, which can reduce the corrosion to equipment and simplify the preparation process; Substituting molecular sieve with long carbon chain quaternary ammonium salt as the support, the catalyst can be placed at the middle interface of water and oil, and the transfer rate of active oxygen atoms can be accelerated.

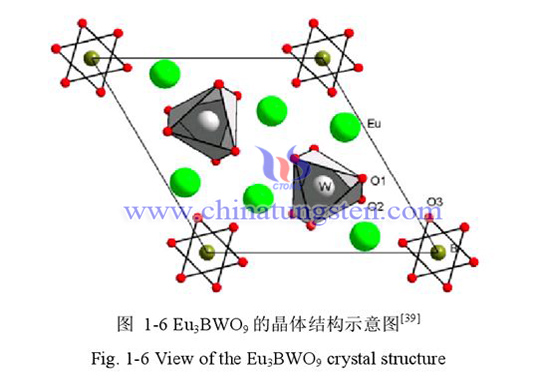
With Na_ 2WO_ 4•2H_ 2O,H_ 3BO_ 3 and Eu (NO_3)_ 3•6H_ Rare earth polyoxometalate K based on borotungstate can be prepared by conventional solution synthesis method using 2O, etc. as raw materials_ (7.5)H_ (4.5)[Eu(BW_(11)O_(39))(W_5O_(18))]•21.5H_ 2O。 Rare earth luminescent materials have been widely used in lighting, laser crystals, military and agricultural fields. Europium ion Eu3+is the main red emitting active ion in rare earth luminescent materials. Luminescent materials based on borate and tungstate have been widely used because of their good physical and chemical stability and good luminescent properties.
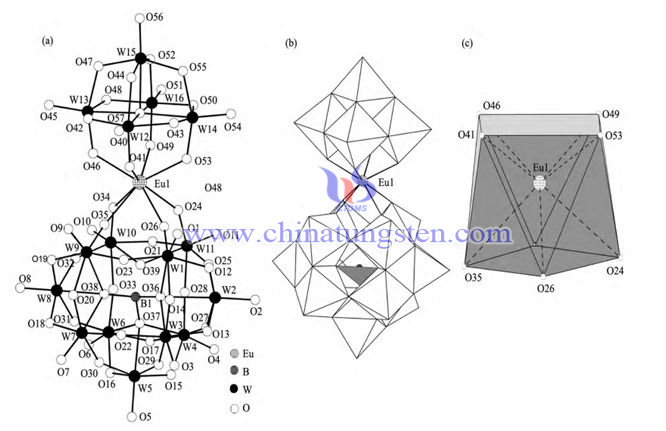
Europium doped borotungstate K7.5 H4.5 [Eu (BW11 O39) (W5O18)] 21.5H2O Anion [Eu (BW11O39) (W5O18)] 12 molecular structure diagram, anion [Eu (BW11O39) (W5O18)] 12 polyhedron, Eu3+coordination environment polyhedron.
A series of rare earth borotungstate phosphors can be synthesized by high temperature solid state method and Pechini method. Potential applications of rare earth borotungstate doped with Eu3+; The crystallographic position of Eu3+in the rare earth borotungstate matrix was determined by position selective excitation spectroscopy and emission spectroscopy; Its potential applications were studied by analyzing its luminescence decay curve, luminescence thermal stability, concentration quenching and luminescence chromaticity. Gd4-xEuxB2WO12 (x=0.2-4.0) rare earth borotungstate phosphor was synthesized by high temperature solid-state method. The analysis of its emission spectrum and excitation spectrum shows that the phosphor can be effectively excited by near ultraviolet/blue light, and can emit strong red light (618nm); The analysis of selective excitation spectra and emission spectra shows that Eu3+ions only occupy a low symmetry, ordered lattice position in the matrix lattice; The concentration and temperature dependence of luminescence on Eu3+concentration showed that the phosphor was concentration quenched.
Rare earth borotungstate phosphor Eu3BWO9 phosphor is a pure phase material with a particle size of about 200 nm; The phosphor can be effectively excited by near ultraviolet/blue light, and is a potential red phosphor for white LED, and its red light intensity is stronger than commercial Y2O2S: Eu3+phosphor; The thermal stability study shows that the luminescence of the phosphor is not affected by temperature and has good thermal stability; The occupation of Eu3+in the matrix lattice is analyzed by laser position selective excitation and emission spectroscopy of Eu3+, which shows that Eu3+occupies a highly ordered, non inversion symmetric lattice position in the matrix lattice.


Gd3 BWO9 crystal belongs to hexagonal crystal system and P63 space group. In Gd3 BWO9 crystal, there is only one Gd lattice, one B lattice and one W lattice. Based on the principle of ionic radius and charge similarity, Bi3+tends to preferentially occupy Gd lattice sites to form Bi luminescence centers. Bismuth doped borotungstate is a green phosphor with strong absorption at 250-400 nm and no absorption in the blue light region, which can effectively avoid the reabsorption between phosphors and has a stable structure. Bismuth doped boron tungstate phosphor is applied to the packaging of full spectrum LED devices including UV or near UV LED chips.
Bismuth doped borotungstate green phosphor and its preparation: The general chemical formula of bismuth doped borotungstate green phosphor is Gd3 x BWO9: xBi3+, where x is the amount of the substance, and 0 < x ≤ 0.3mol. Weigh the compounds containing Gd, B, W and Bi, grind and mix the mixed materials and pre burn them at 400 - 800 ℃ in the air environment for 48h, and then grind and mix them after cooling to room temperature.


LaBWO6 (lanthanum doped borotungstate), as a composite salt of tungstate and borate systems, has excellent physical and chemical properties and is an ideal matrix crystal for activating ion doping. It is precisely because of the excellent properties of this crystal that the growth of large size labwo_6 single crystals has attracted people's research interest for a long time. However, because the crystal structure of this compound has the structural characteristics of both tungstate and borate, the growth raw materials often contain borate and tungstate, and the melt viscosity is high in the high-temperature melting state, and the product is usually glass rather than amorphous, so it is extremely difficult to prepare labwo6 crystal.
According to the molar ratio of labwo6 powder, li2w2o7 powder and b2o3 powder is 1:2:1, and the total weight after melting is 30g, raw materials such as la2o3, h3bo3, li2co3 and wo3 are weighed. The weighed raw materials are fully ground evenly and transferred to a platinum crucible, and placed in a high temperature furnace, rising to 300 ℃ at 2 ℃/min, then rising to 600 ℃ at 1 ℃/min, and then rising to 1050 ℃ at 2.5 ℃/min, and kept at this temperature for 10h, Then, reduce the furnace temperature from 1050 ℃ to 950 ℃ at the rate of 10 ℃/h, and then reduce the furnace temperature to 930 ℃ at the rate of 5 ℃/h, with the platinum wire acting as seed crystal, and then reduce the temperature to 915 ℃ at the rate of 5 ℃/d, and finally rapidly reduce the temperature to room temperature. After the furnace temperature drops to room temperature, lift the seed crystal rod, take out the crucible, take down the colorless and transparent crystal from the platinum wire, clean it with water, and dry it to obtain a size of 15 × fifteen × 0.2 mm3 labwo6 crystal.
The solid optical device system prepared by labwo6 crystal or active ion doped labwo6 crystal can be used in particle detection, laser devices, spectroscopy devices, biomedical or military fields.

Lanthanum doped borotungstate has different names according to different combination modes, such as lanthanum doped borotungstate, lanthanum inserted borotungstate, lanthanum and borotungstate, lanthanum (Ln) substituted borotungstate, lanthanum heteropoly tungstoborate complex.
Heteropoly Tungstoborate Complex Lanthanide is a kind of catalyst with good performance. It also has anti-virus effect, and can be used for lactase separation, cellulose photolysis agent, and manufacture of heat-resistant paint. Synthesis of 11 heteropoly complexes of Ln=La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Ty, Ho, Yb in K15 [Ln (BW11O39) 2]. xH2O.
As a new type of solid light source, white light emitting diode (WLED), compared with traditional lighting sources (incandescent lamp, fluorescent lamp), has many advantages such as high luminous efficiency, low energy consumption, long life, green environmental protection and good reliability, and is known as the next generation of lighting sources. Rare earth doped borate phosphors are a new kind of rare earth fluorescent materials which are being developed to adapt to the development of large screen high-definition color projection television and computer terminal display technology. The doped lanthanum tungstoborate phosphor can avoid the shortcomings of low color rendering index and poor color reduction of blue chip and yellow phosphor, and solve the problems of red, green and blue color mixing ratio control, color reabsorption and energy loss.
![Picture of K15 [Ln (BW11O39) 2]. xH2O Element Analysis Data Sheet Picture of K15 [Ln (BW11O39) 2]. xH2O Element Analysis Data Sheet](img/K15[Ln(BW11O39) 2]xH2O.jpg)
K15 [Ln (BW11O39) 2]. xH2O Element Analysis Data Sheet (%)
A preparation method of doped lanthanum tungstoborate phosphor
Mix La2O3, H3BO3, WO3 and rare earth oxides uniformly, calcine them at 950 ~ 980 ℃, keep them for 8 ~ 10h, and then cool them to prepare La1-x-yBWO6: xSm3+, yDy3+. The rare earth oxides are Sm2O3 and/or Dy2O3, where 0.00 ≤ x ≤ 0.10, 0.00 ≤ y ≤ 0.10, and x and y are different. Under ultraviolet radiation, LaBWO6: Sm3+phosphor emits orange red light, LaBWO6: Dy3+phosphor emits white light, and LaBWO6: Sm3+, Dy3+phosphor emits warm white light. The method is easy to operate, and the calcination temperature is low. The LaBWO6: Sm3+, Dy3+phosphors obtained are single matrix materials, which can be used as phosphors for LED.


As early as 1940s, some scholars studied 11 tungstoborate (POTASSIUM 11 TUNGSTOBORATE) and determined its molecular formula as K9 [BW11O89] 13H2O, or K9 [BW11O89] nH2O. In a wide variety of heteropoly anions, there is a general formula Is [XM11O89] (12-n), where M is W or Mo, X is P, As, S, Ge, B when M=W, or M=Mo is P, As, S, Ge, B. N is the positive charge of X. These anions are classical in structure and properties The general formula [XM12O40] (8-n) - of 12 series heteropolyanions of type A is closely related. They can be obtained by partial degradation of corresponding series of compounds, and in turn, they can form corresponding series of compounds by combining with simple oxyacid ions. The only exception is 11 tungstoborate ion, which is degraded into the same simple oxy acid ion with the corresponding 12 tungstoborate ion under the same acidity. Therefore, it cannot be obtained by partial degradation of 12 series compounds, but can only be obtained by acidification and condensation of simple oxy acid ions. Because such 11 series compounds contain coordination unsaturated oxygen atoms, they are often called "unsaturated" heteropolyanions.
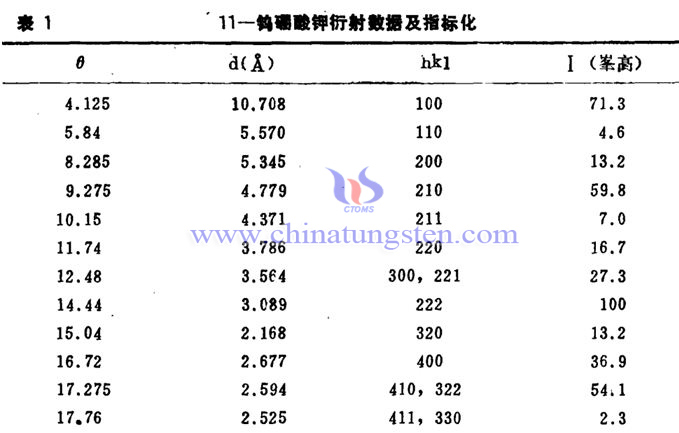
In 1988, some diffraction data and indicators obtained from scholars' research on 11 potassium tungstoborate


English Name: Hexapotassium,borane,hydride,tungsten,vanadium,hentetracontahydrate
CAS No:93253-86-2
Molecular Weight:3066.28
Molecular Formula:BH85K6O41VW11 /(BO40VW11.6K)
